Heat Capacity of Calorimeter
Differential Scanning Calorimeters DSC measure temperatures and heat flows associated with thermal transitions in a material. Knowing the heat capacity of the bomb calorimeter material water and of the fuse wire one can calculate the exact amount of heat released by combustion of the sample.

Specific Heat Capacity Physics Lessons Science Teaching Resources Science Facts
Mass of the calorimeter and stirrer.

. Calorimeter device for measuring the heat developed during a mechanical electrical or chemical reaction and for calculating the heat capacity of materials. To calculate the energy required to raise the temperature of any given substance heres what you require. The water increases in temperature by 10 degrees C.
Specific heat capacity is the most useful quantity available from DSC because it is directly related to sample properties and. The heat capacity in calories per gram is called specific heat. This will require 2669 kJ of heat energy.
It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. All data Tsonopoulos and Ambrose 1995. Heat flow is proportional to the heat difference of heat sink and holders.
Ive been tasked with working out the Latent heat of steam the following are all my results and values used. Calorimeters have been designed in great variety. Joules watt-seconds or joule Newton-meter.
If initially the temperature of the water is 200C and after burning the nuts in the calorimeter we measure a water temperature of 333C then the change in temperature of the water T f - T i equals 133C and the heat captured by the calorimeter Q water is 150 g 0001 Calg C 133C or 20 Cal. Thus the temperature difference between the sample and the. 25772 JK-1 Mass of water and Calorimeter and stirrer.
The entropy and the free energy of formation J. This is the amount of heat required to raise 1 gram of that substance by 1C. I left a 60W light bulb on for 30 days which raised my electric bill by 432 kWh kilowatt-hours.
The definition of the calorie is based on the specific heat of water defined. Specific Heat Capacity c Specific heat capacity of any substance is defined as the amount of heat required to change the temperature of a unit mass of the substance by 1 degree. Place one litre 1 kg of water in the calorimeter.
Soc 1929 51 2738. Energy is power integrated over time. Experiments were carried out using a MicroCal PEAQ-ITC calorimeter with MicroCal PEAQ-ITC Software version 13 both Malvern at 5 C in 20 mM Na 2 HPO 4 pH 75 150 mM NaCl or SPG buffer pH 50.
Properties measured by TA Instruments DSC techniques include glass. The mass of the material m The temperature change that occurs DeltaT The specific heat capacity of the material c which you can look up. A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types.
Heat loss by the fuel is equal to the heat gained by the water. If you have a specific heat capacity in Jg C then you need the mass of the substance in grams. Power is the rate at which work is done or energy is transmitted.
The below-mentioned formula can be used to calculate specific heat capacity values. The vessel is filled with water and the fuel is burned leading to the heating of the water. Therefore specific heat capacity c Qm Delta T.
0068Kg Thermal Capacity of the Calorimeter and Stirrer. Temperature profile from a bomb calorimeter experiment. By knowing the initial mass of the fuel sample the heating value of the sample can be calculated by dividing the heat released by the.
In case the sample occurs endothermic or exothermic phenomena such as transition and reaction this endothermic or exothermic phenomena is compensated by heat sink. An alternative truly measuring at operational conditions lies in using an adiabatic flow calorimeter and thus evaluating the enthalpy balance of a small quantity of the HTF in a bypass of the system 5. If you have it in Jkg C then you need the mass of the substance in kilograms.
The SI unit of specific. The heat capacity of toluene from 14 deg K to 298 deg K. One type in widespread use called a bomb calorimeter basically consists of an enclosure in which the reaction takes place surrounded by a liquid such as water that absorbs.
Heat sink has the enough heat capacity compared to the sample. 0145Kg Mass of water 0077 Thermal capacity of water. A simple calorimeter just consists of a thermometer attached to a.
Unlike differential scanning calorimeters adiabatic flow. Energy is the capacity to do work. We would like to show you a description here but the site wont allow us.
Q mc Delta T. Such measurements can be made easily with this. Place the immersion heater into the central hole at the top of the calorimeter.
The only thing you need to remember is that you have to use consistent units for mass. Common usage includes investigation selection comparison and end-use performance evaluation of materials in research quality control and production applications. Say in a calorimeter a fixed amount of fuel is burned.
Heat capacity ratio of heat absorbed by a material to the temperature change. In the previous article we discussed the specific heat capacity of substances. Clamp the thermometer into the smaller hole with the stirrer.

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Entire C Coffee Cups Chemistry Education Chemistry
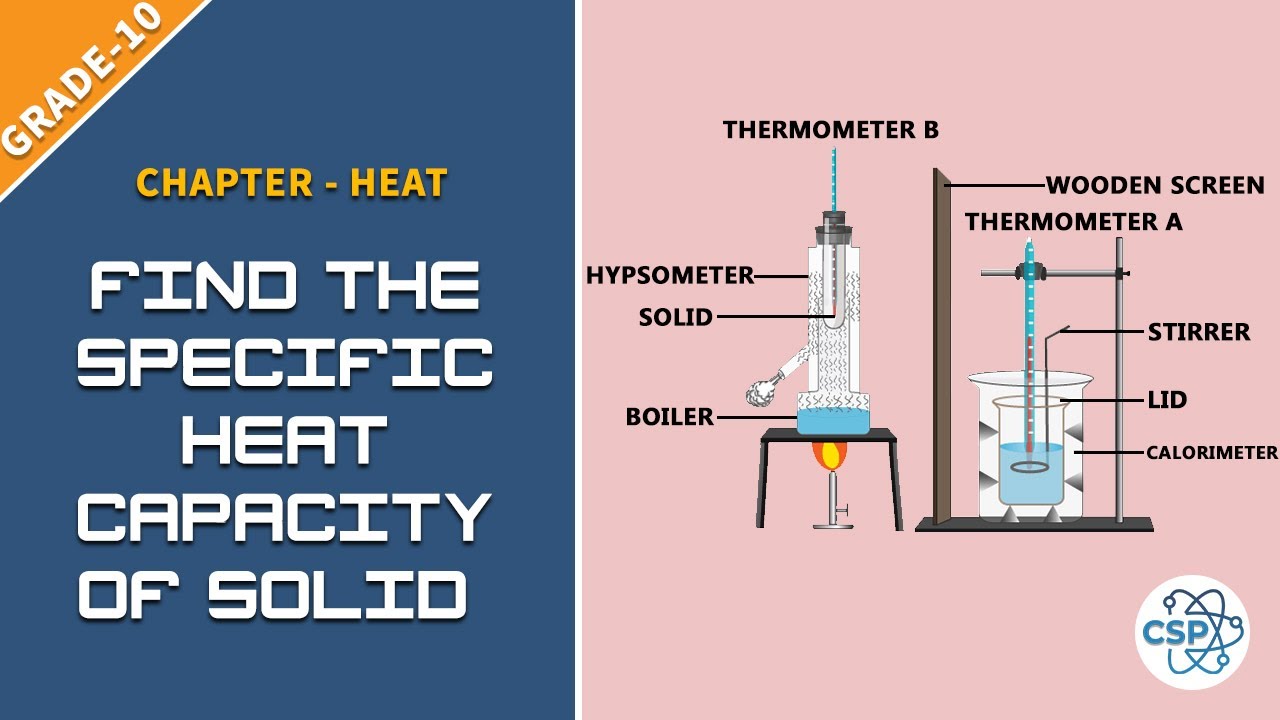
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Worksheets Capacity Worksheets Letter Reversals
Comments
Post a Comment